- 2025,09,15
Recently, Chengyi Pharmaceutical has obtained the GMP re certification certificate for small capacity injections (non final sterilization) issued by the Zhejiang Provincial Food and Drug Administration, covering four specifications: 1ml, 2ml, 5ml, and 10ml, with an annual production of 150 million tubes. The certificate is a series of antiviral drugs and vitamins, and is valid from June 26, 2018 to June 25, 2023.


The GMP "re certification" passed this time covers major varieties such as ribavirin injection and vitamin K1 injection. Public information shows that ribavirin injection is an antiviral drug used for the treatment of viral pneumonia and bronchitis caused by respiratory syncytial virus. The Ribavirin injection produced by Chengyi Pharmaceutical mainly comes in two specifications: 5ml: 0.5g and 1ml: 0.1g, and currently has a high reputation in the domestic market.
Another representative variety is vitamin K1 injection, which is used for vitamin K deficiency. It is used for bleeding caused by vitamin K deficiency, such as bleeding caused by obstructive jaundice, biliary fistula, chronic diarrhea, etc., prothrombin deficiency caused by coumarins, sodium salicylate, etc., neonatal bleeding, and vitamin K deficiency caused by long-term application of spectral antibiotics.
The 101 workshop, which has passed the GMP "re certification" this time, has two small capacity injection production lines, with a filling and sealing machine of 12 needles per unit. Currently, it can produce four specifications: 1ml, 2ml, 5ml, and 10ml. There are 11 small capacity injection products produced year-round, with a designed production capacity of 150 million units.
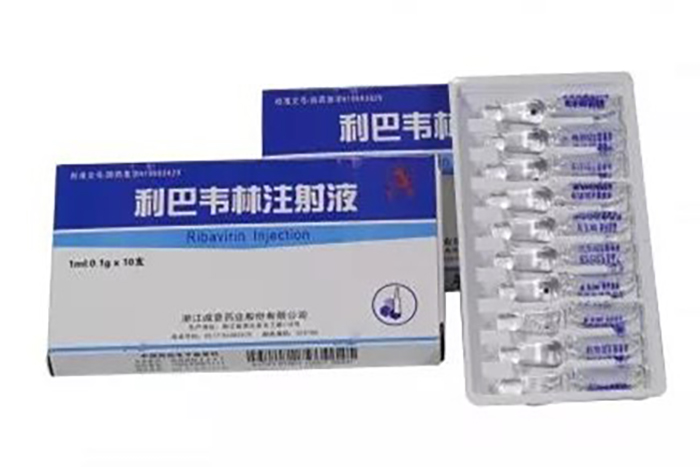
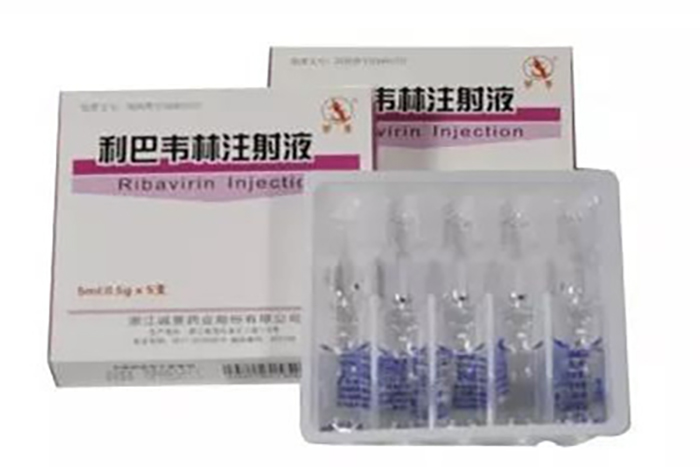

On June 27th, the company announced that this is a "re certification" after the expiration of the original "Drug GMP Certificate". The above-mentioned workshop has passed GMP certification and been issued a "Drug GMP Certificate", indicating that the company's relevant production lines meet the requirements of the new version of GMP. Obtaining a "re certification" for drug GMP is beneficial for the company to maintain stable production capacity and meet market demand.
