Acyclovir can cause acute renal failure. When patients with kidney damage receive acyclovir treatment, it can cause death. When using acyclovir for treatment, it is necessary to carefully observe for signs and symptoms of renal failure (such as oliguria, anuria, hematuria, low back pain, bloating, nausea, vomiting, etc.), and monitor urine routine and renal function changes. Once abnormalities occur, medication should be stopped immediately. Strictly follow the indications and dosage recommended in the instructions to avoid excessive use. When using acyclovir for treatment, sufficient water should be consumed to prevent drug deposition in the renal tubules. Special attention should be paid to the use of acyclovir in patients receiving potentially nephrotoxic drugs, as this may increase the risk of renal dysfunction and increase reversible central nervous system symptoms. Elderly people, pregnant women, and children should use acyclovir with caution or under monitoring.
[Drug Name]
Common name: Acyclovir Capsules
Trade name: Xavier
[Ingredients]
Main ingredient: Acyclovir
Chemical name: 9- (2-hydroxyethoxymethyl) guanine
Chemical structural formula: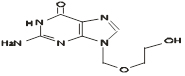
Molecular formula:C8H11N5O3
Molecular weight: 225.21
[Character]The content of this product is white to almost white powder.
[Indication]
1. Herpes simplex virus infection: Used for the initial and recurrent cases of genital herpes virus infection, and for the prevention of recurrent cases, this product is taken orally.
2. Herpes zoster: Oral administration is used for the treatment of mild cases of herpes zoster in individuals with normal immune function and those with immunodeficiency.
3. Treatment of chickenpox in immunocompromised individuals.
[Specifications]0.2g/particle
[Usage dosage]Oral
1. Genital herpes initial treatment and skin mucosal herpes simplex in immunocompromised individuals: 0.2g (1 capsule) per dose, 5 times a day, for a total of 10 days, commonly used in adults; Or 0.4g (2 capsules) at a time, 3 times a day, for a total of 5 days; Recurrent infection: 0.2g (1 capsule) once, 5 times a day, for a total of 5 days; Chronic inhibitory therapy for recurrent infections, 0.2g (1 capsule) once, 3 times a day, for a total of 6 months. If necessary, the dosage can be increased to 5 times a day, 0.2g (1 capsule) once, for a total of 6-12 months.
2. Shingles: 0.8g (4 capsules) per dose is commonly used in adults, 5 times a day, for a total of 7-10 days.
3. Adult patients with renal insufficiency should adjust their dosage according to the following table:
| Creatinine clearance rate (ml/min) (ml/s) | Dose (g) | Administration interval (hours) | |
| Genital herpesInitial or intermittent therapy | 10(0.17) | 0.2 | 4 (5 times a day) |
| 0-10(0~0.17) | 0.2 | 12 | |
| Chronic inhibitory therapy for genital herpes | 10(0.17) | 0.4 | 12 |
| 0-10(0~0.17) | 0.2 | 12 | |
| Herpes zoster | ;25(0.42) | 0.8 | 4 (5 times a day) |
| 10-25(0.17~0.42) | 0.8 | 8 | |
| 0-10(0~0.17) | 0.8 | 12 |
4. Chickenpox: Children over 2 years old should receive 20mg/kg of body weight once, four times a day, for a total of 5 days. If symptoms appear, treatment should be immediately initiated. The commonly used dosage for children and adults above 40kg is 0.8g (4 capsules) once, 4 times a day, for a total of 5 days.
[Adverse reactions]Occasionally experiencing dizziness, headache, joint pain, nausea, vomiting, diarrhea, stomach discomfort, decreased appetite, thirst, decreased white blood cells, mild increase in proteinuria and urea nitrogen, skin itching, etc. Long term administration occasionally results in acne, insomnia, and menstrual disorders.
[Taboo]Prohibited for those who are allergic to this product.
[Note]
1. Individuals who are allergic to ganciclovir may also be allergic to this product.
2. Those who are dehydrated or have liver or kidney dysfunction should use it with caution.
3. Long term or multiple treatments with this product may lead to resistance of herpes simplex virus and herpes zoster virus to this product in patients with severe immune deficiency. If there is no improvement in skin lesions after the application of acyclovir in patients with herpes simplex, the sensitivity of the herpes simplex virus to this product should be tested.
4. Follow up examination: As most patients with genital herpes are prone to cervical cancer, they should be examined at least once a year for early detection.
5.Once symptoms and signs of herpes appear, medication should be administered as soon as possible.
6.Eating has no significant effect on blood drug concentration. However, during the administration period, patients should be given sufficient water to prevent the product from settling in the renal tubules.
7.Intermittent short-term therapy is effective for recurrent genital herpes infection. Due to animal experiments that have found the impact and mutagenicity of this product on fertility, the oral dosage and course of treatment should not exceed the recommended standards. The long-term treatment for recurrent genital herpes should not exceed 6 months.
8.A single hemodialysis can reduce blood drug concentration by 60%, so a dose should be replenished after hemodialysis.
9.This product has no significant effect on the latent infection and recurrence of herpes simplex virus and cannot eradicate the virus.
[Medication for pregnant and lactating women]Drugs can pass through the placenta, although animal experiments have shown no effect on embryos, pregnant women still need to weigh the pros and cons of medication. The concentration of drugs in milk is 0.6-4.1 times that of blood drug concentration. Although no abnormalities have been found in infants, lactating women should use them with caution.
[Pediatric drugs]The dosage for children under 2 years old has not been determined yet.
[Geriatric use]Due to the decline of physiological renal function, the dosage and interval of this product need to be adjusted.
[Drug interaction]
1.Combined with Zidovudine, it can cause nephrotoxicity, manifested as deep lethargy and fatigue.
2.Competing with probenecid to inhibit organic acid secretion, combined use of probenecid can slow down the excretion of this product, prolong its half-life, and accumulate drug volume in the body.
[Drug overdose]Symptomatic treatment and supportive therapy should be used, with sufficient water supply. Hemodialysis helps to excrete drugs from the blood.
[Pharmacology toxicology]Antiviral drugs. It has inhibitory effects on herpes simplex virus, varicella zoster virus, cytomegalovirus, etc. in vitro. After entering cells infected with herpes virus, this product competes with deoxyribonucleoside for viral thymidine kinase or cell kinase. The drug is phosphorylated into an activated acyclovir triphosphate and then inhibits virus replication in two ways: ① interfering with virus DNA polymerase to inhibit virus replication; ② Under the action of DNA polymerase, it binds to the growing DNA strand, causing an interruption in the extension of the DNA strand. This product has a special affinity for viruses, but has low toxicity to mammalian host cells. In vitro cell transformation assay has reported carcinogenicity, but animal experiments have not found any evidence of carcinogenicity. Some animal experiments have shown that high concentrations of drugs can cause mutations, but there is no evidence of chromosomal changes. The carcinogenic and mutagenic effects of this product are not yet clear. High dose injection can cause testicular atrophy and sperm number reduction in animals. The drug can pass through the placenta. Animal experiments have proved that it has no effect on embryos.
[pharmacokinetics]Oral absorption is poor, with about 15% to 30% being absorbed by the gastrointestinal tract. Eating has no significant effect on blood drug concentration. It can be widely distributed in various tissues and bodily fluids, including the brain, kidneys, lungs, liver, small intestine, muscles, spleen, milk, uterus, vaginal mucosa and secretions, cerebrospinal fluid, and herpes fluid. The concentration is high in the kidneys, liver, and small intestine, and the concentration in cerebrospinal fluid is about half that in blood. Drugs can pass through the placenta. After oral administration of 200mg and 400mg every 4 hours, the peak blood concentrations (Cmax) after 5 days were 0.6mg/L and 1.2mg/L, respectively. The protein binding rate of this product is low (9%~33%). Metabolized in the liver, the main metabolites account for 9% to 14% of the administered dose and are excreted through urine. Blood elimination half-life (T1/2 β) About 2.5 hours. When the creatinine clearance rate is 50-80ml/min and 15-50ml/min, the half-life of blood elimination (T1/2) β) 3.0 hours and 3.5 hours respectively. Half life of blood elimination in anuria (T1/2) β) Up to 19.5 hours, reduced to 5.7 hours during hemodialysis. This product is mainly excreted through the kidneys through glomerular filtration and tubular secretion. About 14% of the medication is excreted in its original form through urine, with a fecal excretion rate of less than 2%. The exhaled breath contains trace amounts of medication. Hemodialysis removes approximately 60% of the medication from the blood within 6 hours. Peritoneal dialysis clearance is minimal.
[Storage]Sealed storage.
[Package]Aluminum plastic packaging, 10 capsules/plate × 1 board/box.
[Expiration date]24 months
[Executive standards]Chinese Pharmacopoeia 2010 Edition Part 2
[Approval number]National Pharmaceutical Standards H10940158
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com