- 2025,09,15
Today, the Shanghai Stock Exchange staged a second stop, with an opening price of 18.91 yuan. Only two trades were locked at 22.69 yuan, an increase of 43.97%.
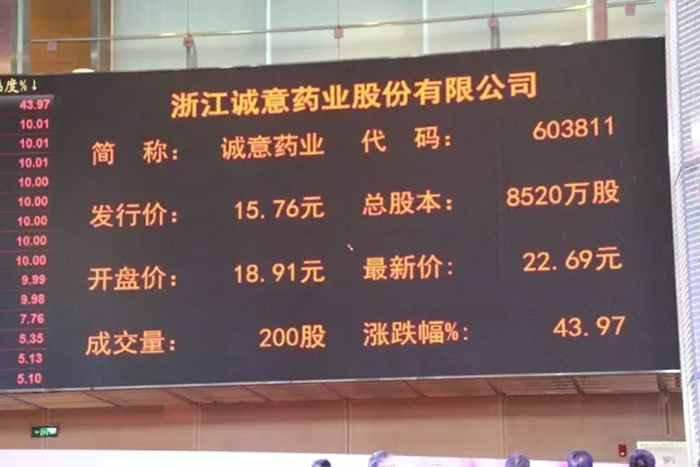
Cheng Yi Pharmaceutical is the first main board listed enterprise in Dongtou District and the first local enterprise listed on the main board of the Shanghai Stock Exchange in Wenzhou in recent two years.
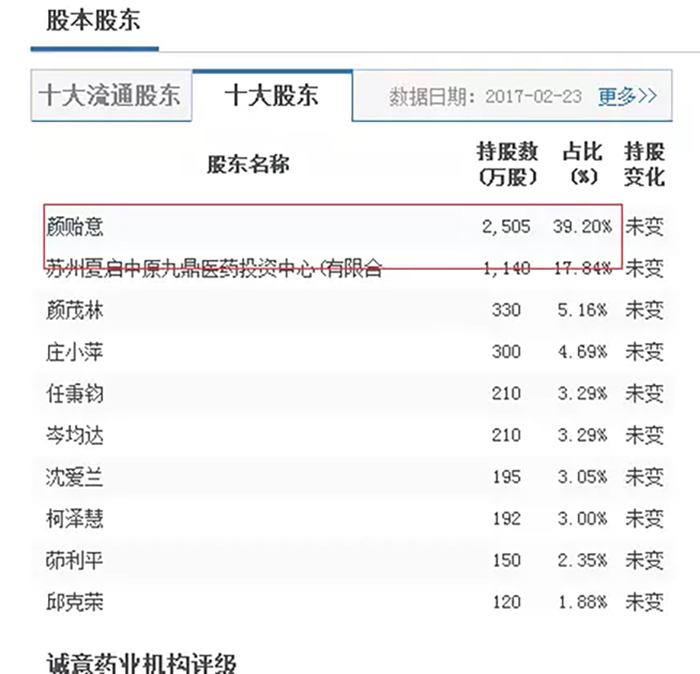
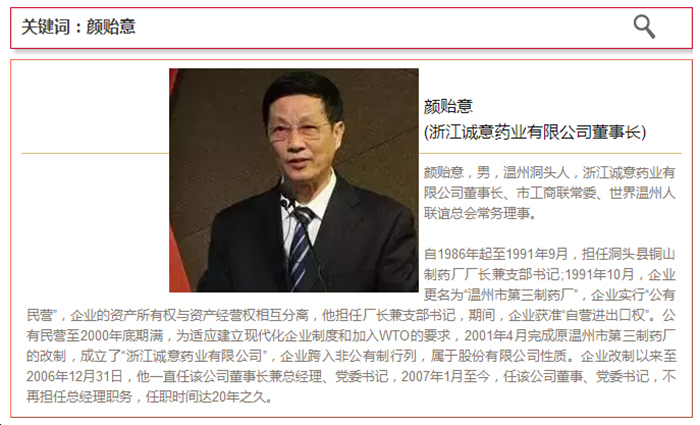
Chairman Yan Yiyi directly holds 25.05 million shares issued, accounting for 39.2% of the company's total share capital. Based on the closing price of 22.69 yuan on that day, his wealth has exceeded 568 million yuan.
Cheng Yi Pharmaceutical, whose predecessor was founded in 1966, is engaged in the research, development, production and sales of drugs. Its therapeutic effects range from arthritis, diuresis, sedation and brain tonic, antiviral and anti-tumor, and its main products include Glucosamine hydrochloride preparations and APIs, tolasemide preparations, Gastrodin APIs, Ribavirin and Azathioprine APIs. Among them, Glucosamine hydrochloride preparations and APIs, tolasemide preparations and Gastrodin APIs mainly focus on the domestic market, and Ribavirin and Azathioprine APIs mainly focus on exports.

The relevant person in charge of the Wenzhou Banking Regulatory Bureau believes that the successful listing of Sincere Pharmaceutical has boosted confidence for enterprises in the region preparing to go public. Enterprises should seize the opportunity of accelerating the pace of IPO issuance, accelerate the integration with the capital market, and use capital strength to strengthen enterprises. Going public not only means achieving multi-channel financing, but also attracts technical talents and brings positive effects to the enterprise.

Cheng Yi Pharmaceutical issued 21.3 million new shares, with a total share capital of 85.2 million shares after the issuance. The raised funds are mainly used for the technical renovation project of the preparation building, the construction project of the R&D center, and the marketing network construction project, with a total construction period of 2-3 years.

After the completion of the project, the enterprise is expected to further expand the market share of preparation products, improve the company's Drug development level and detection capability, strengthen and deepen the distribution of marketing network in the country, and lay a good foundation for the company's sustainable and stable development.

In the next three years, the company's Product development will focus on marine medicine, biomedicine and traditional Chinese medicine. At the same time, the company will carry out extended development of tablets, granules, freeze-dried agents and other dosage forms for existing products such as Ribavirin, Clindamycin phosphate, Glucosamine hydrochloride, etc. In the future, product development will implement the simultaneous development of APIs and supporting preparations.