- 2025,09,15

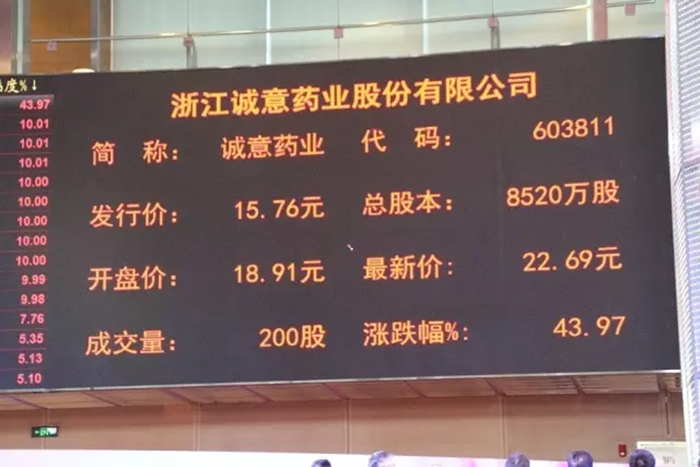
On March 15, Zhejiang Cheng Yi Pharmaceutical Co., Ltd. (hereinafter referred to as "Chengyi Pharmaceutical") held the first listing ceremony on the Shanghai Stock Exchange. The reporter saw on site that Cheng Yi Pharmaceutical's stock price rose to a red trend at the opening, with an opening price of 18.91 yuan. As of the reporter's press release, the stock price had exceeded 22 yuan, an increase of 43.97%.
As a comprehensive modern pharmaceutical enterprise specializing in the production of capsules, injections, APIs and pharmaceutical intermediates, Cheng Yi Pharmaceutical has developed many high-tech products such as Ribavirin, Aciclovir, Azathioprine, Clindamycin, Erythromycin propionate, acetylcysteine, tolasemide and Glucosamine hydrochloride in recent years, The mass production of a series of products from raw materials to injections and capsules for the same variety has achieved good economic benefits.
At present, the company and its subsidiaries have 65 drug production approval numbers. Among them, 43 product specifications were listed in the national medical insurance catalogue, 15 drugs were listed in the National Essential medicines Catalogue (2012 edition), 31 product specifications were produced all year round, and there were 4 national new drug varieties, including 3 Class II new drugs, 1 Class IV new drug, 9 invention patents, and 3 utility model patents.

Under the leadership of the corporate spirit of "reaching the world with sincerity and benefiting people's livelihoods", multiple products under Cheng Yi Pharmaceutical have been awarded titles such as branded products, high-quality pharmaceutical products, and high-quality products. The "Lishan" trademark and "Sankang" trademark have also been recognized as famous trademarks in Zhejiang Province. At the same time, the company has fully passed the Chinese GMP certification, the US FDA, the UK MHRA, the Australian TGA, the EU EMEA, the GMP audit of the Singapore National Drug Administration, and the international standards ISO9001 and ISO14001 systems. Its sales regions cover more than 20 countries and regions such as the United States, Canada, Europe, South Africa, and Southeast Asia, with exports accounting for over 50% of the total sales.
It is understood that Cheng Yi Pharmaceutical will take the opportunity of this public offering of shares to raise funds for the construction of investment projects, further expand the market share of pharmaceutical products, further improve the company's Drug development level and detection capability, strengthen and deepen the distribution of marketing network in the country, and lay a good foundation for the company's sustainable and stable development.

Yan Yiyi, Chairman of Cheng Yi Pharmaceutical, stated at the listing ceremony that, We will adhere to the main development directions of marine medicine, biopharmaceuticals, traditional Chinese medicine, and new formulations, while developing the health industry, implementing marketing reforms, and striving to build the company's core competitiveness. Taking advantage of the opportunity of listing, we will enter the capital market in a planned, step-by-step, and actively and steadily manner through mergers and acquisitions, shareholding, and other forms, so as to extend the industrial chain, achieve leapfrog development of the company, and promote the development of the pharmaceutical industry The increase in national fiscal revenue and the employment of social personnel will make greater contributions.
