- 2025,09,15
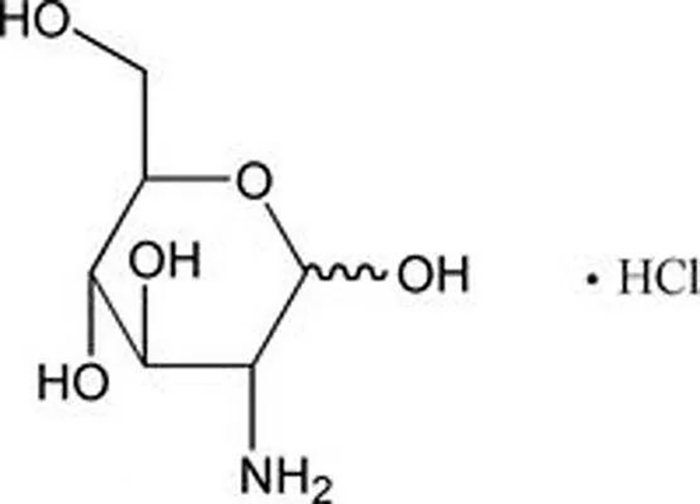
Recently, Zhejiang Cheng Yi Pharmaceutical Co., Ltd. produced glucosamine hydrochloride capsules (three product specifications) that passed the consistency evaluation of generic drugs and obtained the approval for drug supplementation application issued by the National Drug Administration. Among them, 0.48g glucosamine hydrochloride capsule is the first drug in China to complete the consistency evaluation of this product specification, and this enterprise is also the first pharmaceutical enterprise in Wenzhou to pass the consistency evaluation of generic drugs.

Glucosamine hydrochloride is a new generation OTC marine drug for the treatment of osteoarthritis, mainly targeting the etiology of osteoarthritis. It can be used to alleviate and eliminate symptoms such as pain and swelling in osteoarthritis; Glucosamine is a physiological component in the body with good tolerance. Clinical evidence has shown that glucosamine can improve joint activity and alleviate pain within a large therapeutic range, blocking the progression of osteoarthritis, and has become the preferred drug for treating osteoarthritis.

In recent years, the Market Supervision Bureau of Dongtou District has actively promoted the consistency evaluation of generic drugs in pharmaceutical enterprises. In response to the high technical difficulty and large capital investment in the consistency evaluation of generic drugs, it has actively contacted enterprises, organized industry experts to conduct policy promotion and implementation in enterprises, and strengthened the quality and technical guidance on the original research reference formulations, content, related substances, dissolution curves, and other aspects.
At the same time, in response to the situation where enterprises have invested a large amount of funds in the consistency evaluation of generic drugs, Dongtou District has actively introduced the "Special Incentive Policy for the Quality and Efficacy Consistency Evaluation of Generic Drugs", providing financial support of up to 4 million yuan for varieties that have passed the consistency evaluation of generic drugs in the district.
At present, the domestic market share of glucosamine hydrochloride capsules produced by Zheng Cheng Yi Pharmaceutical Co., Ltd. is over 50%, ranking first in domestic sales. This time, glucosamine hydrochloride capsules have successfully passed the consistency evaluation, achieving consistent evaluation results in terms of quality and efficacy with the original research. This not only saves medical expenses for patients and ensures the safety and effectiveness of public medication, but also improves the overall development level of the pharmaceutical industry, creating significant economic benefits and social value.
Source:Zhejiang News Network
