[Drug name]
Common name: Ribavirin capsules
Product name: Vilac
[Ingredients]
Main ingredients: ribavirin
Pseudonym: 1- β- D-furanribosyl-1H-1,2,4-triazol-3-carboxamide.
Chemical structural formula: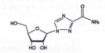
Molecular formula:C8H12N4O5
Molecular weight:244.21
[Character]The content of this product is white or almost white particles or powder.
[Indication]Suitable for viral pneumonia, bronchitis, and skin herpes virus infections caused by respiratory syncytial virus.
[Specifications]0.15g/capsule.
[Usage dosage]
Oral 1. Viral respiratory infection: 0.15g (1 capsule) once in adults, 3 times a day, for a course of 7 days. 2. Skin herpes virus infection: 0.3g (2 capsules) once in adults, 3 times a day, for a course of 7 days. 3. Children should take 10mg/kg of body weight four times a day for a course of 7 days.
[Adverse reactions]Common adverse reactions include anemia, fatigue, etc., which disappear immediately after discontinuing the medication. Rare adverse reactions include fatigue, headache, insomnia, loss of appetite, nausea, vomiting, mild diarrhea, constipation, and can cause a decrease in red blood cells, white blood cells, and hemoglobin.
[Taboo]Prohibited for those who are allergic to this product or pregnant women.
[Note]
1. Those with severe anemia or abnormal liver function should use it with caution.
2. Interference with diagnosis: Oral administration of this product can cause up to 25% increase in blood bilirubin. High doses can cause a decrease in hemoglobin levels.
3. Take medication as soon as possible. The administration of respiratory syncytial virus pneumonia within the first 3 days is generally effective. This product is not suitable for patients who have not been diagnosed with respiratory syncytial virus infection in the laboratory.
4. Long term or high-dose administration may have adverse effects on liver function and blood count.
[Medication for pregnant and lactating women]
1. This product has a strong teratogenic effect. A daily dose of 1mg/kg in rabbits can cause embryonic damage, so it is prohibited for use in pregnant women and women at risk of pregnancy (this product is slowly eliminated in the body and cannot be completely cleared within 4 weeks after discontinuation).
2. A small amount of medication is excreted from milk and is toxic to both mother and child animals. Therefore, lactating women need to pause breastfeeding during the medication period, and milk should also be discarded. Due to the self-limiting nature of respiratory syncytial virus infection in lactating women, this product is not intended for use in such cases.
[Pediatric drugs]The oral dosage for children under 6 years old is uncertain.
[Medication for the elderly]Elderly people do not recommend using it.
[Drug interactions]This product and zidovudine have antagonism at the same time, because this product can inhibit the conversion of zidovudine into active zidovudine phosphate.
[Drug overdose]High dose application can cause heart damage, and can cause breathing difficulties, chest pain, etc. in patients with respiratory diseases (chronic obstructive pulmonary disease or asthma).
[Pharmacology toxicology]
1. Pharmacology
Broad spectrum antiviral drugs. It has the ability to inhibit the growth of various viruses such as respiratory syncytial virus, influenza virus, hepatitis A virus, adenovirus, etc. in vitro, but its mechanism is not fully understood. This product does not alter virus adsorption, invasion, or shelling, nor does it induce the production of interferon. After entering cells infected with the virus, drugs rapidly phosphorylate, and their products act as competitive inhibitors of virus synthase, inhibiting inosine monophosphate dehydrogenase, influenza virus RNA polymerase, and mRNA guanyltransferase, thereby causing a decrease in intracellular guanosine triphosphate, damaging virus RNA and protein synthesis, and inhibiting virus replication and transmission. It may also have immune and neutralizing antibody effects on respiratory syncytial viruses.
2. Toxicology
Animal experiments have found that this product can induce benign tumors in the breast, pancreas, pituitary, and adrenal glands, but its carcinogenicity in humans has not been confirmed. Drugs can cause deformities in the head, palate, eyes, jaw, bones, and gastrointestinal tract of animals such as hamsters, resulting in reduced offspring survival. However, primate experiments have not found any effects of drugs on fetuses. Oral administration of ribavirin was administered to mice, rats, and monkeys at doses of 30, 60, and 120 mg/kg, respectively, or for more than 4 weeks (equivalent to human doses: 4.8, 12.3, and 111.4 mg/kg for children weighing 5 kg, or 2.5, 5.1, and 40 mg/kg for adults weighing 60 kg), resulting in cardiac injury.
[Pharmacokinetics]Oral absorption is rapid, with a bioavailability of about 45%, and a small amount can be inhaled through aerosols. The blood drug concentration reached its peak 1.5 hours after oral administration, with a peak blood drug concentration (Cmax) of approximately 1-2mg/L. Children inhale medication with a mask for 2.5 hours daily for a total of 3 days, with an average peak blood concentration (Cmax) of 0.2mg/L; Aspiration for 20 hours per day for a total of 5 days, with an average peak blood concentration (Cmax) of 1.7mg/L and almost no binding with plasma proteins. The concentration of drugs in respiratory secretions is mostly higher than that in blood. Drugs can enter red blood cells and accumulate in large amounts. After long-term medication, the drug concentration in the cerebrospinal fluid can reach 67% of the blood drug concentration during the same period. This product can penetrate the placenta and also enter milk. Metabolism in the liver. The half-life of blood elimination (t1/2) is approximately 0.5-2 hours. This product is mainly excreted through the kidneys. The urine excretion rate within 72 to 80 hours is 30% to 55%. The 72 hour fecal excretion rate is about 15%. Drugs can accumulate in red blood cells for several weeks.
[Storage]Sealed storage.
[Package]Aluminum plastic packaging, 10 capsules/plate × 1 board (or 2 boards)/box.
[Period validity]24 months
[Executive standards]Chinese Pharmacopoeia 2010 Edition Part 2
[Approval number]National Pharmaceutical Standards H10940157
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com

