[Drug name]
Common name: Tolasemide Capsules
Product name: Lizhi
[Ingredients]
Main ingredients: Tolasemide
Chemical name: N - {[(1-methylethyl) amino] carbonyl} -4- [(3-methylphenyl) amino] -3-pyridinesulfonamide.
Chemical structural formula: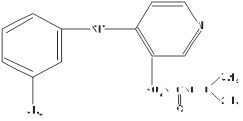
Molecular formula:C16H20N4O3S
Molecular weight:348.43
[Character]This product is a hard capsule with white or almost white particles and powder as the content.
[Indication]Edema caused by congestive heart failure.
[Specifications]10mg/capsule
[Usage dosage]Oral administration, starting at a dose of 10mg (1 capsule) once a day. Depending on the condition, the dosage can be increased to 20mg (2 capsules) once a day.
[Adverse reactions]
Approximately 4000 patients were evaluated for the safety of this product: 800 of them had been using this product for at least 6 months, while over 380 had been using it for more than 1 year. Among these patients, 564 underwent clinical trials of this product in the United States, and 274 served as blank controls. The side effects of this product are usually brief and not related to age, gender, race, or course of treatment. The incidence of discontinuation of treatment due to side effects was 3.5% in the United States, compared to 4.4% in the control group. In the United States and Europe, the rate of discontinuation due to side effects in patients with congestive heart failure is 3.0 (38/1250), while the rate of furosemide is 3.4% (13/380); The withdrawal rate for patients with renal insufficiency was 2.0% (8/409), while furosemide was 4.8% (11/230); The discontinuation rate for liver cirrhosis patients was 7.6% (11/170), and the rate for furosemide was 0%. The most common factors for discontinuing this product are (in descending order of incidence): dizziness, headache, nausea, weakness, vomiting, hyperglycemia, excessive urination, hypokalemia, severe dry mouth, low blood volume, impotence, esophageal bleeding, and indigestion. Due to these side effects, the withdrawal rate ranges from 0.1% to 0.5%. In blank control studies conducted in the United States, more than 1% of side effects that may or are likely to be associated with medication include headache, excessive urination, dizziness, rhinitis, fatigue, diarrhea, abnormal electrocardiogram, cough, constipation, nausea, joint pain, indigestion, sore throat, muscle pain, chest pain, insomnia, edema, and neuroticism. In these clinical trials, the daily dosage range of this product is 1.25mg~20mg, with most patients taking 5mg~10mg for a course of 1-52 days, with an average of 41 days. The above side effects only have a higher incidence of excessive urination in the tolasemide group compared to the control group. In a controlled study of hypertension, the incidence of excessive urination was 1% in the control group, 4% in the tolasemide 5mg/day, and 15% in the 10mg/day group. Excessive urination has not been reported as a side effect event in patients with heart failure and liver and kidney dysfunction treated with this product. The clinical study cannot exclude the serious adverse reactions related to drugs, including atrial fibrillation, chest pain, diarrhea, digitalis poisoning, gastrointestinal bleeding, hyperglycemia, hyperuricemia, hypokalemia, hypotension, hypovolemia, thrombosis, rash, rectal bleeding, syncope and tachycardia. There are reports of a patient experiencing vascular edema after using this product, and it was found that the patient is allergic to sulfonamide drugs. The side effects listed in the clinical controlled study were not evaluated for drug treatment relevance. Arthritis and other non-specific musculoskeletal problems were greater in the tolasemide group than in the control group, but gout was greater in the control group than in the tolasemide group. The incidence and severity of these side effects are not related to the dosage of tolasemide. One patient stopped taking this product due to myalgia, while another patient in the control group stopped taking it due to gout. Hypokalemia: See precautions.
[Taboo]
1. Patients who are known to be allergic to tolasemide or sulfonylurea drugs should not use this product.
2. Patients without urine should not use this product.
[Note]
1. Due to sudden changes in body fluid and electrolyte balance, liver coma may occur. Patients with liver cirrhosis and ascites should use this product with caution. It is best for such patients to start using this product (or any other diuretic) in the hospital. To prevent hypokalemia and metabolic alkalosis, it is best to use this product together with aldosterone antagonists or drugs with low potassium excretion.
2. Ototoxicity: Tinnitus and hearing loss (usually recoverable) were observed after rapid intravenous injection of other loop diuretics or oral administration of this product. It is not sure that these adverse reactions are related to this product. In animal experiments, ototoxicity can be observed at extremely high plasma concentrations of tolasemide. When intravenous injection, it should be administered slowly for at least 2 minutes, and the dosage of a single medication should not exceed 200mg.
3. Body fluid volume and electrolyte consumption: patients using diuretics can observe electrolyte imbalance, hypovolemia or pre renal azotemia, which may cause one or more of the following symptoms: dry mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pain or spasm, fatigue, hypotension, oliguria, tachycardia, nausea, vomiting. Excessive diuretic effects may cause dehydration, decreased body fluid volume, thrombosis or embolism (especially in elderly patients). For patients with body fluid and electrolyte imbalance, hypovolemia, and pre renal azotemia, laboratory examination can observe that blood sodium increases or decreases, blood chlorine increases or decreases, blood potassium increases or decreases, acid and base levels are abnormal, and blood urea nitrogen increases. If the above symptoms occur, discontinue this product until symptoms recover and reuse it at low doses.
4. In a controlled trial conducted in the United States, hypertensive patients who took this product (5-10mg/day) for 6 weeks showed an average decrease of approximately 0.1mEq/L in blood potassium. At any time during the treatment process, the percentage of patients with blood potassium levels below 3.5mEq/L in the experimental group (1.5%) was similar to that in the placebo group (3%). After taking the medication for 1 year, there was no further change in the average blood potassium level of the patient. The incidence of dose-dependent hypokalemia is higher in patients with congestive heart failure, cirrhosis, or kidney disease when the dosage of this product is higher than in the United States antihypertensive trial. Hypokalemia induced by diuretics is a risk factor for arrhythmia in patients with cardiovascular diseases, especially those who use digitalis. Patients with liver cirrhosis, those who receive diuretics quickly, those with insufficient electrolyte intake, and those who also use corticosteroids or adrenocorticotropins are at the highest risk of developing hypokalemia. Patients who use tolasemide need to regularly monitor their blood potassium and other electrolytes.
5. Other electrolytes:
(1) Calcium: A single use of this product in healthy volunteers resulted in an increase in urinary calcium excretion, but in a 4-6 week trial of hypertensive patients, the patient's blood calcium levels only slightly increased. In a long-term trial of patients with congestive heart failure, the average one-year decrease in blood calcium levels was 0.10mg/dL (0.02mmol/L). Among 426 patients treated with this product for 11 months, hypocalcemia was not reported as an adverse reaction.
(2) Magnesium: After a single use of this product in healthy volunteers, the excretion of magnesium in urine increased, but in the 4-6 week trial of hypertension patients, their blood magnesium levels only slightly increased. In a long-term trial of hypertensive patients, the average one-year increase in blood magnesium levels was 0.03mg/dL (0.01mmol/L). Among the 426 patients treated with this product for 11 months, only one case of hypomagnesemia (1.3mg/dL, equivalent to 0.53mmol/L) was reported as an adverse reaction. In a long-term trial of patients with congestive heart failure, it was estimated that the average one-year increase in blood magnesium levels was 0.2mg/dL (0.08mmol/L), but due to the use of magnesium supplements by many patients, these data are unclear. In a 4-week trial, patients did not use magnesium supplements, and the percentage of patients with blood magnesium levels below 1.7mg/dI (0.70mmol/L) in the tolasemide 5mg and 10mg groups was 6% and 9%, respectively.
6. Blood biochemistry:
(1) Blood urea nitrogen, creatinine, and uric acid: Tolasemide slightly increased the above parameters in a dose-dependent manner. After taking 10mg of this product daily for 6 consecutive weeks, hypertensive patients' blood urea nitrogen increased by an average of 1.8mg/dL (0.6mmol/L), serum creatinine increased by an average of 0.05mg/dL (4mmol/L), and serum uric acid increased by an average of 1.2mg/dL (70mmol/L). After long-term medication, the above parameters can further undergo mild changes, but can be restored after discontinuation. Patients who use this product can develop symptomatic gout, but the incidence is similar to that of the placebo group.
(2) Blood glucose: After taking 10mg of this product daily, the blood glucose level of hypertensive patients increased by an average of 5.5mg/dL (0.3mmol/L) after 6 weeks. In the following year, the blood glucose level further increased by 1.8mg/dL (0.1mmol/L). In the long-term trial of patients with diabetes, there was no significant change in the average fasting blood glucose level compared with the base value. There have been reports of cases of elevated blood sugar, but it is not common.
(3) Lipids: In a short-term controlled trial conducted in the United States on hypertensive patients, after taking 5mg, 10mg, and 20mg of tolasemide daily, the total plasma cholesterol levels of patients increased by 4, 4, and 8mg/dL (0.10-0.20mmol/L), respectively. Symptoms disappeared after long-term medication.
(4) In the same short-term trial of hypertensive patients, daily intake of tolasemide 5mg, 10mg, and 20mg resulted in an average increase in plasma triglyceride levels of 16, 13, and 71mg/dL (0.15-0.80mmol/L), respectively. In a long-term trial, patients took 5-20mg of this product daily. After one year of treatment, no clinically significant changes were observed in their blood lipid levels compared to the baseline.
7. In the long-term trial of hypertensive patients, the experimental group showed a slight decrease in hemoglobin, hematocrit, and red blood cell count, while the white blood cell count, platelet count, and serum alkaline phosphatase levels slightly increased. Despite significant statistical differences, these changes are not of significant medical significance. Except for alkaline phosphatase, no significant change trend was observed in the experiments on other anhydrases.
[Medication for pregnant lactating women]
1. Pregnant women: No embryonic toxicity or teratogenic effects were found in doses up to 5mg/kg/day (in mg/kg and body surface area, equivalent to 15 and 10 times the human dose of 20mg) for rats, and up to 1.6mg/kg/day (in mg/kg and body surface area, equivalent to 5 and 1.7 times the human dose of 20mg) for rabbits. When the doses of rabbits and rats increase by 4 and 5 times, the toxicity of fetuses and mothers includes average weight loss Increased absorption of fetuses and delayed ossification of fetuses. Because no sufficient control test has been conducted in pregnant women, and the results of reproductive toxicity test on animals do not always predict the reaction to human body, pregnant women must weigh the advantages and disadvantages when taking this product.
2. Breastfeeding women: It is currently unknown whether this product can be secreted in human milk. Due to the fact that many drugs can be secreted in human milk, lactating women should use this product with caution.
[Pediatric drugs]The efficacy and safety data for children taking this product have not yet been established, and caution should be exercised when using this product. After taking another type of loop diuretic in premature infants, edema caused by patent ductus arteriosus and hyaline membrane disease was observed, occasionally related to renal calcification. Stones were sometimes almost invisible on X-ray films, but sometimes filled in the renal pelvis in a deer horn shape. Some stones can disappear by themselves, and the incidence of hypercalciuria decreases after chlorothiazide is combined with other loop diuretics. Other premature infants with hyaline membrane disease who take another loop diuretic have an increased risk of persistent patent ductus arteriosus, which may be related to the mediating effect of prostaglandin E. No research has been conducted on the use of this product in such patients.
[Medication for the elderly]In clinical trials conducted in the United States, 24% of patients are over 65 years old, and approximately 4% of patients are over 75 years old. The results showed that the efficacy and safety of taking this product in elderly patients did not show any age-related differences compared to young patients.
[Drug interactions]
1. Patients with primary hypertension will combine this product with β The combination of receptor blockers, ACE inhibitors, and calcium channel blockers, as well as the use of this product with digitalis, ACE inhibitors, and nitrates in patients with congestive heart failure, did not result in any new or unexpected adverse reactions.
2. This product has no effect on the binding rate of glibenclamide, warfarin, and plasma protein, and has no effect on the anticoagulant effect of phenylpropanoid coumarin (related coumarin derivatives). It also has no effect on digoxin or carvedilol (vasodilators), β The pharmacokinetics of receptor blockers were not affected. Healthy volunteers used this product in combination with spironolactone, which resulted in a decrease in renal clearance and an increase in AUC value. However, clinical experience has shown that there is no need to adjust the dosage of the two drugs.
3. Due to the competition between salicylic acid drugs and this product for renal tubular secretion, salicylic acid toxicity can be observed in the high-dose group of salicylic acid when combined with this product. Although the interaction between this product and non-steroidal anti-inflammatory drugs has not been studied, the combination of these drugs with furosemide can occasionally lead to renal dysfunction.
4. Like many diuretics, indomethacin partially inhibits the diuretic sodium excretion effect of this product. The above phenomenon can be observed in patients with limited sodium intake (50mEq/day), but not in patients with normal sodium intake (150mEq/day).
5. Both cimetidine and spironolactone have no effect on the pharmacokinetics and diuretic effects of this product. Taking digoxin simultaneously increases the AUC value of this product by 50%, but there is no need to adjust the dosage of this product.
6. No studies have been conducted on the interaction between the combination of this product and colonemide in humans. However, in animal experiments, colonemide has reduced the absorption rate of this product orally, and it is not recommended to use the combination of the two drugs.
7. Simultaneously taking probenecid reduces the amount of secretion of this product into the proximal tubules, resulting in a decrease in its diuretic effect.
8. It is known that other diuretics can reduce the renal clearance rate of lithium and increase the risk of lithium toxicity, so the combination of the two types of drugs must be used with caution. No research has been conducted on the drug interaction after the combination of this product and lithium.
9. Other diuretics can increase the potential ototoxicity of aminoglycoside antibiotics and etaniac acid, especially in patients with renal function injury. No study has been conducted on the interaction between this product and the above drugs.
[Drug overdose]At present, there is no report of overdose of this product, but if the product is overdosed, the expected symptoms include dehydration, hypovolemia, hypotension, hyponatremia, hypokalemia, hypochloremic alkalosis, and blood concentration. After taking excessive amounts of this product, fluid and electrolyte supplementation should be used. The method for determining the serum concentration of this product and its metabolites in the laboratory has not yet been widely applied. There is no data indicating that some physiological measures such as adjusting the pH of urine can promote the elimination of this product and its metabolites. Hemodialysis cannot clear this product.
[Pharmacology toxicology]Pharmacological effects: This product is mainly used in the thick segment of the ascending branch of the medullary loop to inhibit the Na+/K+/2Cl - transport system. Clinical pharmacological studies have also confirmed that this is the site of action of this product in the human body, and it has no effect on other parts of the kidney. Therefore, the diuretic effect of this product is more closely related to the drug's excretion rate in the urine than its concentration in the blood. Tolasemide can increase the excretion of sodium, chlorine, and water in urine, but does not significantly alter glomerular filtration rate, renal blood flow, and acid-base balance. Toxicological effects; The carcinogenic effect of tolasemide was not significantly increased when administered to rats and mice at doses of 9mg/kg/day and 32mg/kg/day, respectively. This dose is equivalent to a human 20mg dose of 27-96 times (in mg/kg) or 5-8 times (in body surface area). In the rat experiment, the high-dose group of female mice found a significant increase in the incidence of renal tubular injury, renal interstitial inflammation, renal adenoma, and renal cancer, but the incidence of tumors was not higher than the historical control. In animal experiments, other diuretics such as furosemide and hydrochlorothiazide also found similar non neoplastic renal injury in the high-dose group. Mutagenic effect; In various in vivo and in vitro experiments, tolasemide and its main metabolites in the human body have no mutagenic effects. The above tests include Ames test (with or without S9), human lymphocyte chromosome aberration test and sisters chromatid exchange test, hamster bone marrow micronucleus aberration test, mouse and rat unscheduled DNA synthesis test, etc. Reproductive toxicity Tolasemide 25 mg/kg/day (in mg/kg and body surface area, the dose is 75 times and 13 times of the dose of 20 mg for human use, respectively) has no effect on the reproductive capacity of female and male rats. No fetal toxicity or teratogenicity was observed in rats treated with 5mg/kg/day of tolasemide (in mg/kg and body surface area, the doses were 15 and 10 times that of human 20mg/day, respectively) and rabbits treated with 1.6mg/kg/day of tolasemide (in mg/kg and body surface area, the doses were 5 and 1.7 times that of human 20mg/kg/day, respectively). Fetal and maternal toxicity (average weight loss, increased absorption of placentas, and delayed fetal ossification) can be observed when administered to rats at 4 times or rabbits at 5 times higher doses, respectively.
[Pharmacokinetics]The bioavailability of tolasemide is about 80%, with small individual differences, and a 90% confidence limit of 75% -89%. The absorption of the drug is little affected by first-pass metabolism. Within 1 hour after oral administration, the serum concentration reaches its peak (Cmax), with a dosage range of 2.5-200mg. The Cmax and AUC values of this product are proportional to the dosage. Taking medication at the same time as eating delayed the peak time of the blood concentration of this product by about 30 minutes, but the total bioavailability (AUC value) and diuretic effect remained unchanged. The absorption of this product is basically unaffected by liver and kidney dysfunction. The distribution volume of this product in healthy adults, patients with mild to moderate renal failure, and congestive heart failure is 12-15L, and the distribution volume in patients with liver cirrhosis is approximately doubled. The half-life of this product in healthy volunteers is approximately 3.5 hours. After taking this product, the liver metabolism rate and urine excretion rate of patients with normal kidney function are about 80% and 20%, respectively. The main metabolites of this product in the human body are carboxylic acid metabolites without pharmacological activity. The two secondary metabolites may have some diuretic effects, but due to metabolic reasons, their diuretic effects cannot be displayed. The plasma protein binding rate of tolasemide is very high (> 99%), and the amount of tolasemide that enters the renal tubules through glomerular filtration is very small. Most of the tolasemide cleared by the kidney is mainly actively secreted into the renal tubules through the proximal glomerular tubules. In patients with decompensated congestive heart failure, the liver and kidney clearance rates decreased after the use of tolasemide, possibly due to reduced liver congestion and renal plasma flow, respectively. The total clearance rate of tolasemide is approximately 50% of that of healthy volunteers, with an increase in plasma half-life and AUC values. Due to the reduced renal clearance rate, only a small amount of this product can enter the site of action in the medullary loop, so its sodium excretion effect on patients with congestive heart failure is lower than that of healthy volunteers. The renal clearance rate of renal failure patients significantly decreased after using this product, but there was no significant change in the overall plasma clearance rate. Only a small amount of this product can enter the action site within the medullary loop, so the sodium excretion effect decreases. If kidney failure patients take high-dose of this product, they can still obtain diuretic effects. Due to the fact that it remains in its original form after elimination through liver metabolism, the total plasma clearance rate and elimination half-life of this product in patients with renal function impairment remain normal. The distribution volume, plasma half-life, and renal clearance rate of liver cirrhosis patients increased after using this product, but the total clearance rate remained unchanged. Except for the decrease in renal clearance rate associated with decreased renal function in elderly patients (but the total plasma clearance rate and elimination half-life remain unchanged), the pharmacokinetics of tolasemide in healthy elderly subjects are similar to those in young subjects.
[Storage]Shade, seal, and store in a dry place.
[Package]Aluminum plastic packaging, 10 capsules/plate × 1 board/small box (or 2 boards/small box).
[Period validity]Tentative 24 months.
[Executive standards]YBH06002005
[Approval number]National Pharmaceutical Standards H20050526
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com