[Drug Name]
Common name: Cytidine Sodium Injection
[Ingredients]
Main ingredient: citicoline sodium
Chemical name: Monosodium salt of choline cytosine nucleoside diphosphate
Structural formula: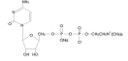
Molecular formula: C14H25N4NaO11P2
Molecular weight: 510.31
All auxiliary materials: None
[Character]This product is a colorless and clear liquid.
[Indications]Coenzyme, used for acute traumatic brain injury and postoperative consciousness disorders.
[Specification]2ml:0.25g
[Usage dosage]Intravenous infusion: 0.25-0.5g (1-2 doses) per day, diluted with 5% or 10% glucose injection, and slowly administered every 5-10 days as a course of treatment; Simple intravenous injection: 100-200mg each time. Intramuscular injection: 0.1-0.3g per day, divided into 1-2 injections.
[Adverse reactions]This product has no obvious toxic effects on humans or animals, and has no effect on respiration, pulse, or blood pressure. Occasionally, there may be transient blood pressure drops, insomnia, excitement, and fever after administration. It can disappear after stopping the medication.
[Taboo]This experiment was not conducted and there are no reliable references available.
[Precautions]High doses should not be used during the acute phase of cerebral hemorrhage. Intramuscular injection is generally not used, and if used, the injection site should be frequently changed.
[Medication for pregnant lactating women]This experiment was not conducted and there are no reliable references available.
[Children's medication]This experiment was not conducted and there are no reliable references available.
[Elderly medication]This experiment was not conducted and there are no reliable references available.
[Drug interactions]There is no information on the interaction between this product and other drugs.
[Drug overdose]This experiment was not conducted and there are no reliable references available.
[Pharmacology Toxicology]This product is a nucleoside derivative that promotes brain substance metabolism and improves cerebral circulation by reducing cerebrovascular resistance and increasing cerebral blood flow. In addition, it can enhance the function of the ascending activation system of the brainstem reticular structure, enhance the function of the pyramidal system, improve motor paralysis, and thus have a certain effect on promoting the recovery of brain function and promoting awakening.
[Pharmacokinetics]Injecting this product can quickly enter the bloodstream, with some entering the brain tissue through the blood-brain barrier. Among them, the choline part becomes a good methylation donor in the body and can transmethylate various compounds, with about 1% of choline being excreted from the urine.
[Storage]Shading and sealed storage.
[Packaging]Ampoule packaging, 10 pieces/box
[Validity period]36 months
[Executive Standards]Chinese Pharmacopoeia 2010 Edition Part 2
[Approval number]National Pharmaceutical Standards H19999550
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com