[Drug Name]
Common name: Terazosin Hydrochloride Capsules
[Ingredients]
Main ingredient: terazosin hydrochloride
Chemical name: 1- (4-amino-6,7-dimethoxy-2-quinolinyl) -4- (tetrahydrofuran-2-formyl) piperazine hydrochloride dihydrate.
Chemical structural formula: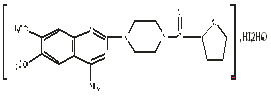
Molecular formula:C19H25N5O4·HCl·2H2O
Molecular weight:459.94
[Character]The content of this product is white or almost white particles or powder.
[Indications]Terazosin hydrochloride can be used to treat benign prostatic hyperplasia. Terazosin hydrochloride can also be used to treat hypertension, either alone or in combination with other antihypertensive drugs such as diuretics or β- Adrenaline blocker combination.
[Specification]2mg/capsule
[Usage dosage]If terazosin hydrochloride is discontinued for a few days, it should be re treated with the first dose regimen.
Benign prostatic hyperplasia
1. The first dose is 1mg (half a pill), taken before bedtime. During the first administration, the patient should be closely monitored to avoid severe hypotension reactions.
2. The maintenance dose should gradually increase to 2mg (1 capsule), 5mg (2.5 capsules), or 10mg (5 capsules) once a day until satisfactory symptoms and/or flow rate improvement are achieved.
3. Commonly used dose 10mg (5 capsules), once a day. It is currently unclear whether higher doses of 20mg (10 capsules) per day can be used for treatment of patients who are not suitable or have no response for 4-6 weeks. If the medication is discontinued for a few days or longer, treatment should be restarted using the first dose regimen.
4. When combined with other antihypertensive drugs, especially calcium channel blocker verapamil, special care should be taken to avoid obvious hypotension and reduce the dosage of this product.
Hypertension
1. The first dose is 1mg (half a pill), taken before bedtime. During the first administration, the patient should be closely monitored to avoid severe hypotension reactions.
2. The maintenance dose should be slowly increased until satisfactory blood pressure is achieved. The recommended dosage is usually 1mg (half a capsule) to 5mg (2.5 capsules) once a day; However, some patients may only be effective at a daily dose of 20mg (10 capsules). Doses higher than 20mg (10 capsules) do not seem to further affect blood pressure, and doses higher than 40mg (20 capsules) have not been studied yet. Blood pressure should be monitored during the administration interval. If the antihypertensive effect decreases after 24 hours of administration, a twice daily administration plan can be considered. If the medication is discontinued for a few days or longer, treatment should be restarted using the first dose regimen. In clinical trials, except for the first medication before bedtime, other medication times should be in the morning.
[Adverse reactions]
1. Six clinical trials of terazosin and blank control for benign prostatic hyperplasia showed that terazosin 1 to 20mg, once daily, had an adverse reaction rate of at least 1%, which was higher than the blank control group, or had clinically significant adverse reactions, including weakness, orthostatic hypotension, dizziness, drowsiness, nasal congestion/rhinitis, and impotence. The incidence of urethral infection was significantly reduced in the terazosin group (Table 1). The risk of adverse events related to hypotension is greatest during the first 7 days of treatment, including various dosing intervals. Table 1: The adverse reactions and events that occurred during the blank control trial for the treatment of benign prostatic hyperplasia are usually brief and mild to moderate, and treatment must be interrupted when severe adverse reactions occur. In the blank control trial, there was no significant difference in the proportion of premature discontinuation of treatment due to adverse reactions between the blank and terazosin groups.
2. A clinical trial of four blank control groups of terazosin in the treatment of hypertension showed that terazosin 1-40mg once a day, alone or in combination with other antihypertensive drugs, showed a significant increase in adverse reactions compared to the blank control group, including weakness, blurred vision, dizziness, nasal congestion, nausea, peripheral edema, palpitations, and drowsiness. In controlled or publicly available short-term or long-term clinical trials, at least 1% of 1987 patients who took medication experienced or reported adverse reactions after marketing, including systemic reactions such as chest pain, facial edema, fever, abdominal pain, neck pain, and shoulder pain; Cardiovascular system: arrhythmia, vasodilation; Digestive system: constipation, diarrhea, dry mouth, indigestion, bloating, vomiting; Metabolic/nutritional disorders: gout; Muscle and iliac system: joint pain, arthritis, joint disease, myalgia; Nervous system: anxiety, insomnia; Respiratory system: bronchitis, cold symptoms, nosebleeds, flu symptoms, worsening cough, pharyngitis, rhinitis; Skin and its accessories: itching, rash, sweating; Special sensations: visual abnormalities, conjunctivitis, tinnitus; Urogenital system: urinary frequency, urinary incontinence (mainly seen in postmenopausal women), and urinary tract infections. After listing, it was shown that there were occasional cases of allergic reactions in patients and reports of abnormal penile erection. Adverse reactions are usually mild or moderate, but sometimes severe and treatment must be interrupted.
[Taboo]This product is contraindicated for individuals who are allergic to terazosin.
[Precautions]
Prostate cancer and BPH cause many of the same symptoms. These two diseases often coexist. So it is believed that patients with BPH should undergo examination before treatment with terazosin hydrochloride to rule out the possibility of prostate cancer.
2. Orthostatic hypotension Although syncope is the most severe orthostatic effect of terazosin, other hypotensive symptoms such as dizziness and palpitations are more common, and in clinical trials of hypertension, 28% of patients experience this symptom. In the BPH trial, 21% of patients experienced one or more of the following symptoms: dizziness, hypotension, orthostatic hypotension, syncope, and dizziness. Patients should be informed that this product may cause syncope and orthostatic hypotension, especially at the beginning of treatment, and avoid driving or dangerous work 12 hours after the first administration, increasing the dosage, or interrupting treatment before resuming use. When symptoms of hypotension occur, it is recommended that the patient sit or lie down, although these symptoms are not always upright, and caution should also be exercised when the patient stands up from a sitting or lying position. If the symptoms of dizziness, dizziness, or palpitations make you feel uncomfortable, you should inform your doctor so that you can consider adjusting the dosage.
3. Patients should be informed that treatment with terazosin may cause drowsiness or drowsiness, and those who must drive or operate heavy machinery should be cautious.
4. Patients should be informed that using terazosin hydrochloride or other similar supine treatment may cause abnormal penile erection. Patients should be aware that this reaction is quite rare, but if not promptly noticed by doctors, it may lead to permanent erectile dysfunction (impotence).
5. Laboratory experiments observed in controlled clinical trials that terazosin slightly reduced hematocrit, hemoglobin, white blood cells, total protein mass, and albumin, but it was statistically significant. This suggests that terazosin has the possibility of causing blood dilution.
[Medication for pregnant lactating women]In studies conducted before and after childbirth in rats, the 120mg/(kg · day) (>75 times the recommended maximum dose for humans) dose group showed significantly higher mortality rates among young rats within 3 weeks after childbirth compared to the control group. It is not yet clear whether terazosin is secreted in breast milk. Because many drugs are secreted in breast milk, attention should be paid to the administration of terazosin to lactating women.
[Children's medication]The safety and effectiveness of this product for children have not been determined yet.
[Elderly medication]This experiment was not conducted and there are no reliable references available.
[Drug interactions]In a controlled trial, terazosin was added to diuretics and certain β- No unexpected interactions were observed in adrenergic blockers. Terazol has been administered in combination with the following types of drugs: analgesics/anti-inflammatory drugs, antibiotics, anticholinergic/parasympathetic drugs, anti gout drugs, cardiovascular drugs, corticosteroids, gastrointestinal drugs, hypoglycemic drugs, sedatives and tranquilizers. When terazosin and verapamil are administered together with other drugs, the average AUC0-24 of terazosin increases by 11% when verapamil is given for the first time, and increases by 24% after three weeks of treatment with verapamil. At this time, the average Tmax of terazosin decreases from 1.3 hours to 0.8 hours. No obvious influence on verapamil was found. When the combination of terazosin and captopril reaches steady state, the maximum plasma concentration of terazosin increases linearly with dose.
[Drug overdose]Excessive use of terazosin hydrochloride can lead to hypotension. Patients can be kept in a supine position to restore blood pressure and normal heart rate. If this method is ineffective, it should be expanded by supplementing body fluids. If necessary, use vasopressors and monitor and maintain renal function. Laboratory data shows that the plasma binding rate of terazosin is 90-94%; Therefore, dialysis treatment may not be beneficial for drug overdose.
[Pharmacology Toxicology]
l. Pharmacodynamics A: The symptoms related to benign prostatic hyperplasia (BPH) and BPH involve bladder outlet obstruction, which includes two basic components: the static part and the dynamic part. The static part is the result of prostate enlargement. For a period of time, the prostate gland will continue to expand. However, clinical studies have shown that the size of the prostate is not related to the severity of BPH symptoms or the degree of urethral obstruction. The dynamic part is a function of increased tension in the smooth muscles of the prostate and bladder neck, leading to narrowing of the bladder outlet. Smooth muscle tension is caused by α It is mediated by the sympathetic stimulation of 1-neneneba adrenergic receptor, which is abundant in prostate, prostate sac and bladder neck. Symptom reduction and improvement in urine flow rate after administration of terazosin in relation to bladder neck and prostate α The relaxation of smooth muscle caused by blocking of 1- adrenergic receptor is related. Because there are relatively few α 1- adrenergic receptor, so terazosin can alleviate the obstruction of bladder outlet without affecting the contraction of bladder. Trazosin was studied in 1222 male patients with BPH symptoms. In three blank control studies, symptom evaluation and urine flow measurement were performed approximately 24 hours after administration. Quantify symptoms using the Boyarsky Index. A questionnaire was used to evaluate symptoms of obstruction (hesitation and discontinuity in urination, dripping urine after urination, size and pressure damage of urine flow, feeling of incomplete bladder emptying) and stimulation (nocturia, daytime frequency of urination, urgency, and difficulty urinating). The 9 symptoms were scored 0-3 for each, with a total score of 27. The analysis of the impact of terazosin on individual urination symptoms showed that compared to the blank, terazosin significantly improved urination hesitation, discontinuity, size and pressure damage of urine flow, feeling of incomplete bladder emptying, drip urination after urination, diurnal urination frequency, and nocturia. A comprehensive evaluation of the overall function and symptoms of urination was conducted, and compared with patients who received blank treatment, patients treated with terazosin showed significant (p ≤ 0.001) overall improvement. In long-term trials, terazosin significantly improved symptoms and maximum urinary flow rate scores, indicating that terazosin relaxes smooth muscle cells. Despite blocking α The 1-neneneba adrenergic receptor also reduces the blood pressure of hypertensive patients caused by the increase of peripheral vascular resistance, but the male patients with BPH with normal blood pressure did not cause clinically significant blood pressure reduction when they were treated with terazosin. B. Hypertension in animals, terazosin reduces blood pressure by reducing total peripheral vascular resistance. The vasodilation and blood pressure lowering effects of terazosin seem to be mainly caused by α It is caused by blocking of 1-neneneba adrenergic receptor. Within 15 minutes after administration, terazosin gradually reduced blood pressure. Patients with mild (approximately 77%, diastolic blood pressure 95-105mmHg) or moderate (approximately 23%, diastolic blood pressure 105-115mmHg) hypertension should receive terazosin once or twice a day at a total dose of 5-20mg/day for clinical trials. Same as all α The same as the antagonist, because terazosin can make the blood pressure drop rapidly after the first or previous administration, the starting dose is 1mg, and then adjusted to a fixed dose or a specific blood pressure endpoint (usually the diastolic pressure in the supine position is 90mmHg). Blood pressure was measured at the end of the dosing interval (usually 24 hours), and the results showed that the antihypertensive effect persisted throughout the entire interval. Typically, the decrease in systolic blood pressure in the supine position was 5-10mmHg greater than in the blank, and the decrease in diastolic blood pressure was 3.5-8mmHg greater. After 24 hours of administration, the heart rate remained unchanged. The amount of blood pressure reaction is similar to that of prazosin, but lower than that of chlorothiazide. Trazosin small dose group significantly reduced patients' total cholesterol, low-density lipoprotein and very low-density lipoprotein in statistics, but did not significantly change high-density lipoprotein and triglyceride. 2. Toxicological carcinogenesis, mutagenicity and reproductive toxicity Terazosin has no mutagenic effect. There is also no evidence to support the carcinogenic effect of terazosin. Treatment does not affect the weight and morphology of the testicles. When administered at doses of 30 and 120mg/(kg · day), the uterine smear showed a decrease in sperm content compared to the control smear, and there was a good correlation between the number of sperm and subsequent conception. When orally administered to rats at doses of 40 and 250mg/(kg · day) (29 and 175 times the recommended maximum dose for humans) for one or two years, the incidence of testicular atrophy significantly increased, but did not increase at doses of 8mg/(kg · day) (>6 times the recommended maximum dose for humans). In the dog experiment, testicular atrophy was also observed at a dose of 300mg/(kg · day) (>500 times the recommended maximum dose for humans) for 3 months, but no testicular atrophy was observed at a dose of 20mg/(kg · day) (>38 times the recommended maximum dose for humans). The teratogenic effects during pregnancy have not been adequately and well controlled in pregnant women, and the safety of terazosin during pregnancy has not been determined. It is not recommended to use terazosin during pregnancy unless it is proven that its benefits outweigh the risks to the mother and fetus.
[Pharmacokinetics]Male patients are basically completely absorbed after taking the medication. Taking medication immediately after meals has minimal impact on absorption, but delays the peak time of plasma concentration by approximately 40 minutes. The liver first-pass metabolism of terazosin is very small. After taking the medication, it reaches its peak about 1 hour and has a half-life of about 12 hours. In the study of the effect of age on the pharmacokinetics of terazosin, it was found that patients aged ≥ 70 years and 20-39 years had plasma half-lives of 14.0 and 11.4 hours, respectively. The plasma protein binding rate is 90-94%. About 40% is excreted through urine, and about 60% is excreted with feces.
[Storage]Shading and sealed storage.
[Packaging]Aluminum plastic packaging, 14 pieces/plate × 1 board/box (or 2 boards/box)
[Validity period]24 months
[Executive standards]WS1-(X-088)-2003Z-2011
[Approval number]National Pharmaceutical Standards H19991110
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com