[Drug name]
Common name: Acetylglutamide Injection
[Ingredients]
Main ingredient: Acetylglutamide
Chemical name: N2 acetyl-L-glutamine.
Chemical structural formula: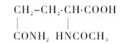
Molecular formula: C7H12N2O4
Molecular weight: 188.18
Auxiliary materials: sodium hydroxide, injection water.
[Character]This product is a colorless and clear liquid.
[Indications]Used for coma caused by traumatic brain injury, coma caused by neurosurgery surgery, liver coma and hemiplegia, high paraplegia, sequelae of poliomyelitis, neurological headache, and low back pain.
[Specification]5ml:0.25g
[Usage dosage]
Intramuscular injection: 100-600mg per day; Reduce the dosage for children or follow medical advice.
Intravenous infusion: Dilute 100-600mg each time with 250ml of 5% or 10% glucose solution and slowly drip. Reduce the dosage for children or follow medical advice.
[Adverse reactions]No adverse reactions have been reported yet.
[Taboo]Prohibited for those who are allergic to any ingredients in this product.
[Precautions]
1. Intravenous infusion may cause a decrease in blood pressure, and attention should be paid when using it.
2. Prohibit the use of drugs when their properties change.
[Medication for pregnant lactating women]This experiment was not conducted and there are no reliable references available.
[Children's medication]Children should reduce the dosage or follow medical advice when using this product.
[Elderly medication]There is currently no research data on the use of this product in elderly patients. Please refer to [Usage and Dosage] and other items.
[Drug interactions]This experiment was not conducted and there are no reliable references available.
[Drug overdose]There is no research data or literature report on drug overdose of this product. Once an overdose occurs, symptomatic and supportive treatment should be given.
[Pharmacology toxicology]Acetylglutamine is an acetyl compound of glutamine that breaks down into glutamic acid and Υ- Aminobutyric acid (GABA). Glutamate participates in information transmission in the central nervous system. Υ- Aminobutyric acid can antagonize the excitatory toxicity of glutamate, improve neural cell metabolism, maintain neural stress ability, reduce blood ammonia, and improve brain function.
[Pharmacokinetics]This product is widely distributed in the body, with high concentrations in the brain, liver, and kidneys, and can penetrate the bloodcerebrospinal fluid barrier. Ammonia is broken down in renal tubular cells and converted into acetylglutamate. Ammonia is secreted and excreted through renal tubules, and acetylglutamate is absorbed and participates in metabolism in the body.
[Storage]Shading and sealed storage.
[Packaging]Ampoule packaging, 5 pieces/box
[Validity period]24 months
[Executive standards]Chinese Pharmacopoeia 2010 Edition Part 2
[Approval number]National Pharmaceutical Standards H33022064
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com