[Drug Name]
Common name: Lincomycin Hydrochloride Injection
[Ingredients]
Main ingredient: Lincomycin hydrochloride
Chemical name: 6- (1-methyl-trans-4-propyl-L-2-pyrrolidinylformamide) -1-thio-6,8-dideoxy-D-red formula- α- D-galactopyranoside hydrochloride hydrate.
Chemical structural formula: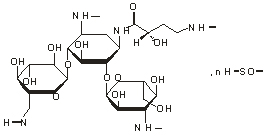
Molecular formula: C18H34N2O6S · HCI · H2O
Molecular weight: 461.02
All auxiliary materials: benzyl alcohol
[Character]This product is a colorless or almost colorless clear liquid.
[Indications]This product is suitable for respiratory infections, skin and soft tissue infections, female reproductive tract infections, pelvic infections, and abdominal infections caused by sensitive Staphylococcus, Streptococcus, Streptococcus pneumoniae, and anaerobic bacteria. The latter two diseases can be used alone or in combination with other antibiotics according to the situation. In addition, for patients with indications for the use of penicillin, such as those who are allergic to or unsuitable for penicillin, this product can be used as a substitute drug.
[Specification]Calculate 2ml: 0.6g based on C18H34N2O6S
[Usage dosage]Intramuscular injection: 0.6-1.2g (1-2 doses) per day for adults, administered in batches. Intravenous infusion: Generally, 0.6g (1 tube) is given once in adults, every 8 or 12 hours, and every 0.6g is dissolved in 100-200ml of infusion for 1-2 hours. Children should weigh 10-20mg/kg daily. It should be noted that every 0.6g is dissolved in at least 100ml of solution during intravenous infusion, and the infusion time should not be less than 1 hour. Infants under 4 weeks old are not required.
[Adverse reactions]
1. Gastrointestinal reactions: symptoms such as nausea, vomiting, abdominal pain, diarrhea, etc; Severe cases include abdominal colic, abdominal tenderness, severe diarrhea (watery or purulent), accompanied by fever, abnormal thirst, and fatigue (pseudomembranous enteritis); Diarrhea, enteritis, and pseudomembranous enteritis can occur in the early stages of medication or several weeks after discontinuation.
2. Hematology: Occasional occurrences of leukopenia, neutropenia, neutropenia, and thrombocytopenia are rare in aplastic anemia.
3. Allergic reactions: visible rash, itching, occasional urticaria, angioneurotic edema, and serum disease reactions. There are few reports of epidermal detachment, bullous dermatitis, erythema multiforme, and S-J syndrome.
4. There have been occasional reports of jaundice caused by the use of this product.
5. During rapid infusion of this product, hypotension, electrocardiogram changes, and even cardiac and respiratory arrest may occur.
6. Intravenous administration causes thrombophlebitis.
[Taboo]Patients with a history of allergies to lincomycin and clindamycin are contraindicated.
[Precautions]
1. When allergic to this product, it is possible to also be allergic to clindamycin.
2. Interference with diagnosis: Serum alanine aminotransferase and aspartate aminotransferase may increase after medication.
3. The following situations should be used with caution:
(1) Individuals with intestinal diseases or a previous history, especially ulcerative colitis, localized colitis, or antibiotic related colitis (this product can cause pseudomembranous colitis).
(2) Decreased liver function.
(3) Severe decline in renal function.
4. During the medication period, close attention should be paid to the frequency of bowel movements. If there is an increase in bowel movements, attention should be paid to the possibility of pseudomembranous enteritis, and medication should be stopped in a timely manner and appropriate treatment should be taken.
5. To prevent the occurrence of acute rheumatic fever, the course of treatment for hemolytic streptococcal infection with this type of drug should be at least 10 days.
6. Treatment of pseudomembranous enteritis caused by this product may recover in mild patients after discontinuation of medication, while in moderate to severe patients, water and electrolyte disorders need to be corrected. If there is no significant improvement in the condition after the above treatment, metronidazole 250-500mg should be taken orally three times a day. If the recurrence occurs, metronidazole can still be taken orally, but if it still does not work, vancomycin (or norvancomycin) can be used orally. Adults should take 0.5-2.0g daily, divided into 3-4 doses.
7. Occasionally, it can lead to excessive proliferation of insensitive microorganisms or cause secondary infections. Once secondary infections occur, corresponding measures need to be taken.
8. Those with a history of asthma or other allergies should use it with caution.
9. For those with longer treatment courses, regular liver and kidney function tests and blood routine tests are required.
[Medication for pregnant lactating women] After passing through the placenta, this product can be concentrated in the fetal liver. Although there have been no reports of problems with human use, it is necessary to fully weigh the pros and cons of its use in pregnant women. This product can be secreted into breast milk, and lactating women should also use it with caution. If necessary, breastfeeding should be suspended.
[Children's medication]Infants less than 1 month old should not use it. This product contains benzyl alcohol and is prohibited for intramuscular injection in children.
[Elderly medication]Elderly people with severe underlying diseases are prone to adverse reactions such as diarrhea or pseudomembranous enteritis, and close observation is necessary when taking medication.
[Drug interactions]
1. It can enhance the neuromuscular blockade of inhalation anesthetics, leading to skeletal muscle weakness and respiratory suppression or paralysis (apnea). Attention should be paid when using it during or after surgery. Treatment with anticholinesterase drugs or calcium salts is expected to be effective.
2. When used in combination with anti peristaltic and anti diarrhea drugs, as well as anti diarrhea drugs containing white clay, this product may cause pseudomembranous enteritis accompanied by severe watery diarrhea during the course of treatment or even several weeks after the treatment. Anti peristaltic and anti diarrhea drugs should not be used in combination as they can delay the excretion of colonic endotoxins, leading to prolonged and aggravated diarrhea. When used in combination with anti diarrhea drugs containing white clay, the absorption of the former will be significantly reduced, so both should not be taken at the same time and should be taken at a certain interval (at least 2 hours).
3. This product has a neuromuscular blocking effect, and when combined with anti myasthenic drugs, it will weaken the effect of the latter on skeletal muscles. To control the symptoms of myasthenia gravis, the dosage of anti myasthenic drugs should be adjusted when combined.
4. Chloramphenicol or erythromycin can replace this product at the target site or inhibit the binding of the latter to the 50S subunit of bacterial ribosomes. In vitro experiments have shown that lincomycin has an antagonistic effect on erythromycin, so lincomycin should not be used in combination with chloramphenicol or erythromycin.
5. When used in combination with opioid analgesics, the respiratory inhibitory effect of this product and the central respiratory inhibitory effect of opioids may cause prolonged respiratory inhibition or respiratory paralysis (apnea) due to the accumulation phenomenon. Therefore, close observation or monitoring of the patient is necessary.
6. This product can enhance the effect of neuromuscular blockers, and the combination of the two should be avoided.
7. There are incompatibilities when intravenous infusion with neomycin and kanamycin in the same bottle.
[Drug overdose]When there is an overdose of medication, it is mainly symptomatic and supportive therapy, such as fluid replacement.
[Pharmacology toxicology]This product has high antibacterial activity against common aerobic Gram positive bacteria, such as Staphylococcus aureus (including those resistant to penicillin G), Staphylococcus epidermidis β Streptococcus hemolyticus, Streptococcus viridis, and Streptococcus pneumoniae. It has good antibacterial effects on anaerobic bacteria, including tetanus bacteria, diphtheria corynebacterium, and gas producing capsule bacteria. It has no activity against Gram negative bacteria such as Enterococcus, meningococcus, Neisseria gonorrhoeae, and Haemophilus influenzae, as well as fungi. This product has no cross resistance to penicillin, chloramphenicol, cephalosporins, and tetracyclines, but partially cross resistance to macrolides. This product acts on the 5OS subunit of sensitive bacterial ribosomes, preventing the extension of peptide chains, thereby inhibiting protein synthesis in bacterial cells. It is generally an antibacterial agent, but at high concentrations, it also has a bactericidal effect on certain bacteria.
[Pharmacokinetics]Adult intramuscular injection of 600mg, reaching the peak blood concentration (Cmax) within 30 minutes. After absorption, it is widely and rapidly distributed in various bodily fluids and tissues, including bone tissue, except for cerebrospinal fluid. It can quickly enter the fetal circulation through the placenta, and the concentration in fetal blood can reach 25% of the maternal blood concentration. The protein binding rate ranges from 77% to 82%. This product is metabolized in the liver and some metabolites have antibacterial activity. The half-life of blood elimination (t1/2) is 4-6 hours, and when liver and kidney functions decrease, t1/2 is extended to 10-20 hours. This product can be excreted through the bile duct, kidneys, and intestines. After intramuscular injection, 1.8% to 24.8% of the drug is excreted through the urine, and after intravenous infusion, 4.9% to 30.3% is excreted through the urine. This product can also be secreted into milk. Hemodialysis and peritoneal dialysis cannot clear lincomycin.
[Storage]Sealed storage.
[Packaging]Ampoule packaging, 10 pieces/box
[Validity period]24 months
[Executive Standards]Chinese Pharmacopoeia 2010 Edition Part 2
[Approval number]National Pharmaceutical Standards H33021519
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com