[Drug name]
Common name: Glucosamine Hydrochloride Capsules
Product name: Vilgu
[Ingredients]
Main ingredient: Glucosamine hydrochloride.
Chemical name: 2-Amino-2-deoxy-D - (+) - pyranoglucose hydrochloride
Chemical structure: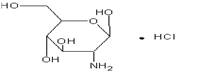
Molecular formula:C6H13NO5·HCl
Molecular weight:215.63
All auxiliary materials: None.
[Character]
This product is a hard capsule with a white powder content.
[Function category]
This product is an over-the-counter analgesic drug.
[Indications]
Used to treat and prevent osteoarthritis in all parts of the body, including the knee, shoulder, hip, wrist, neck, spine, and ankle joints. It can alleviate and eliminate symptoms such as pain and swelling in osteoarthritis, and improve joint mobility.
[Specification]0.24 g
[Usage dosage]
Take 1-2 capsules orally, 3 times a day, and the general course of treatment is 4-12 weeks. If necessary, the medication can be extended under the guidance of a doctor. Repeat treatment 2-3 times a year.
[Adverse reactions]
Rare and mild gastrointestinal discomfort, such as nausea, constipation, bloating, and diarrhea; Some patients may experience allergic reactions, including rashes, itching, and skin redness.
[Taboo]
Prohibited for those who are allergic to this product.
[Precautions]
1. This product should be taken during or after meals to reduce gastrointestinal discomfort, especially for patients with gastric ulcers.
2. Patients with severe liver or kidney dysfunction should use it with caution.
3. After one course of medication, if the symptoms do not improve, please consult a doctor or pharmacist.
4. Pregnant and lactating women should use it with caution.
5. It is prohibited for those who are allergic to this product, and should be used with caution for those with allergic constitution.
6. Do not use this product when its properties have changed.
7. Please keep this product out of reach of children.
8. If using other medications, please consult a doctor or pharmacist before using this product.
[Drug interactions]
1. This product can increase the absorption of tetracycline drugs in the gastrointestinal tract and reduce the absorption of oral penicillin or chloramphenicol.
2. Patients who are taking non-steroidal anti-inflammatory drugs at the same time may need to reduce the dosage of this product or reduce the dosage of non-steroidal anti-inflammatory drugs.
3. There may be interactions between this product and diuretics, and when taking both drugs simultaneously, it may be necessary to increase the dosage of diuretics.
4. If used together with other drugs, drug interactions may occur. For more information, please consult a doctor or pharmacist.
[Storage]Shade, seal, and store in a dry place.
[Packaging]
Aluminum plastic packaging: 10 capsules per plate, 1 or 2 plates or 3 or 4 plates per small box.
Polyethylene bottle packaging: 42 or 90 capsules, 180 capsules, 240 capsules, or 300 capsules per bottle.
[Validity period]Tentative 24 months.
[Executive Standards]YBH11482006
[Approval number]National Pharmaceutical Standards H20060748
[Revision date of instruction manual]July 9th, 2013
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com

