[Drug Name]
Common name: Gastrodia elata injection
[Ingredients]
Main ingredient: Gastrodia elata extract
Chemical name: 4-hydroxymethylbenzene- β- D-glucopyranoside
Chemical structural formula: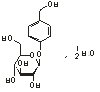
Molecular formula: C13H18O7 · 12 H2O
Molecular weight: 295.38
All auxiliary materials: None
[Character]This product is a colorless and clear liquid.
[Indications]Used for symptoms such as neurasthenia, neurasthenia syndrome, and vascular nerve headache (such as migraine, trigeminal neuralgia, greater occipital neuralgia, etc.), as well as for traumatic brain syndrome, dizziness such as Meniere's disease, drug-induced dizziness, traumatic dizziness, sudden deafness, vestibular meridian inflammation, vertebrobasilar artery insufficiency, etc.
[Specification]2ml:0.2g
[Usage dosage]Intramuscular injection, once 0.1g~0.2g (half to one branch), 1-2 times a day. Organic diseases can be dosed appropriately or as directed by a doctor.
[Adverse reactions]A few patients may experience symptoms such as dry mouth and nose, dizziness, and stomach discomfort, but this does not affect their ability to receive medication and does not require special treatment.
[Taboo]Prohibited for those who are allergic to this product.
[Precautions]Prohibit the use of drugs when their properties change.
[Medication for pregnant lactating women]It is not yet clear.
[Children's medication]This experiment was not conducted and there are no reliable references available.
[Elderly medication]This experiment was not conducted and there are no reliable references available.
[Drug interactions]There is no information on the interaction between this product and other drugs.
[Drug overdose]This experiment was not conducted and there are no reliable references available.
[Pharmacology toxicology]
1. Pharmacology: Pharmacological experiment shows that ephedrine can restore the imbalance between excitation and inhibition of the cerebral cortex, and produce central inhibitory effects such as sedation, sleeping and analgesia.
2. Toxicology: Acute toxicity experiment: Mice were orally or intravenously injected with gastrodin at a dose of 5g/kg. After 3 days of observation, no poisoning or death was observed.
Subacute toxicity experiment: After 4-6 days of administration in dogs and mice, blood tests showed no effect on red blood cell, white blood cell, and platelet counts. Blood assay has no effect on GPT, NPN and cholesterol. Microscopic examination of tissue sections from the heart, lungs, spleen, liver, kidneys, stomach, and intestines of animals showed no cell degeneration. The above results indicate that gastrodin has no effect on the hematopoietic system, liver, kidney function, and blood lipids.
[Pharmacokinetics]After injection, the blood drug concentration was consistent with the sedative effect time, with a elimination half-life of 4.44 hours. The highest distribution in the body is in the kidneys, followed by the liver, lungs, heart, spleen, and brain. Mainly excreted from urine, the total amount excreted from urine, feces, and bile is 76.8% of the administered dose, of which 97% is excreted in urine, mainly in the first 2 hours, with little bile and feces excreted.
[Storage]Keep sealed and stored at room temperature.
[Packaging]Ampoules, 10 pieces/box
[Validity period]36 months
[Executive standards]WS1-XG-023-2001
[Approval number]National Pharmaceutical Standards H33022310
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com