Please read the instruction manual carefully and use it under the guidance of a doctor!
[Drug Name]
Common name: Vitamin K1 Injection
[Ingredients]
Main ingredients: Vitamin K1
Chemical name: 2-methyl-3- (3,7,11,15-tetramethyl-2-hexadecenyl) -1,4-naphthalene
A mixture of trans and cis isomers of diketones.
Chemical structural formula: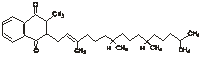
Molecular formula: C31H46O2
Molecular weight: 450.71
All auxiliary materials: Tween-80, propylene glycol, sodium acetate, acetic acid, and sodium pyrosulfite.
[Character]This product is a yellow liquid.
[Indications]Used for bleeding caused by vitamin K deficiency, such as obstructive jaundice, biliary fistula, chronic diarrhea, etc., low prothrombin blood caused by coumarins, sodium salicylate, etc., neonatal bleeding, and vitamin K deficiency in the body caused by long-term use of broad-spectrum antibiotics.
[Specification]lml:10mg
[Usage dosage]1. Hypothrombogenemia: Intramuscular or deep subcutaneous injection of 10mg (1 tube), 1-2 times a day, with a total of no more than 40mg (4 tubes) within 24 hours. 2. Prevention of neonatal bleeding: Mother can be given intramuscular injection or slow intravenous injection of 2-5mg 12-24 hours before delivery. 0.5-1mg can also be injected intramuscularly or subcutaneously in newborns after birth, and can be repeated after 8 hours. 3. When this product is used for intravenous injection in critically ill patients, the administration speed should not exceed 1mg/min.
[Adverse reactions]Occasional allergic reactions. Intravenous injection too fast, exceeding 5mg/min, can cause facial flushing, sweating, bronchospasm, tachycardia, hypotension, etc. There have been reports of rapid intravenous injection leading to death. Intramuscular injection can cause local redness, swelling, and pain. Newborns may experience hyperbilirubinemia, jaundice, and hemolytic anemia after using this product.
[Taboo]Prohibited for severe liver disease or poor liver function.
[Precautions](1) For patients with liver function damage, the efficacy of this product is not significant, and blind dosage can exacerbate liver damage. (2) This product has no effect on the bleeding tendency caused by heparin. There is no need to use this product for traumatic bleeding. (3) This product should be used for intravenous injection slowly, and the administration rate should not exceed 1mg/min. (4) This product should avoid freezing. If there are oil droplets or layering, it should not be used. However, it can be heated to 70-80 ℃ in a dark environment, shaken to allow it to cool naturally. If foreign objects are visible and normal, it can still be used.
[Medication for pregnant lactating women]This product can pass through the placenta, so it should be avoided as much as possible for pregnant women during childbirth.
[Children's medication]Neonatal hemorrhage; Intramuscular or subcutaneous injection, 1mg each time, can be repeated after 8 hours.
[Elderly medication]This experiment was not conducted and there are no reliable references available
[Pharmacological interactions]After mixing this product with phenytoin sodium for 2 hours, particle precipitation can occur, while mixing with vitamin C, vitamin B12, and dextran can easily cause turbidity. When combined with oral anticoagulants such as coumarins, the effects cancel each other out. Salicylic acids, sulfonamides, quinine, and quinidine also affect the effectiveness of vitamin K1.
[Drug overdose]High or excessive doses of drugs can exacerbate liver damage.
[Pharmacology toxicology]This product is a vitamin based medicine. Vitamin K is necessary for liver synthesis factors Ⅱ, Ⅶ, Ⅸ and Ⅹ. The deficiency of vitamin K can cause the disorder or abnormality of the synthesis of these coagulation factors, and the bleeding tendency and prothrombin time can be prolonged clinically.
[Pharmacokinetics]Intramuscular injection takes effect 1-2 hours, and the hemostatic effect is significant 3-6 hours. After 12-14 hours, the prothrombin time returns to normal. This product is metabolized in the liver and excreted through the kidneys and bile.
[Storage]Shaded, sealed, and protected from freezing.
[Packaging]Ampoule packaging, 10 pieces/box
[Validity period]36 months
[Executive standards]Chinese Pharmacopoeia 2010 Edition Part 2
[Approval number]National Pharmaceutical Standards H33020864
[Manufacturing enterprise]
Enterprise Name:Zhejiang Cheng Yi Pharmaceutical Co., Ltd.
Production Address: No.118 Huahua Road, Dongtou County, Zhejiang Province, China
Phone number:86-0577-6348-3979
Fax number:86-0577-6348-5135
Website:en.chengyipharma.com

